Lower gastrointestinal bleeding (LGIB) accounts for approximately 20-33% of episodes of gastrointestinal (GI) hemorrhage, with an annual incidence of about 20-27 cases per 100,000 population in Western countries. However, although LGIB is statistically less common than upper GI bleeding (UGIB), it has been suggested that LGIB is underreported because a higher percentage of affected patients do not seek medical attention.
[1] Indeed, LGIB continues to be a frequent cause of hospital admission and is a factor in hospital morbidity and mortality LGIB is distinct from UGIB in epidemiology, management, and prognosis.
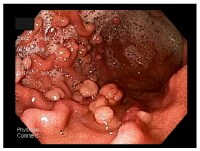
LGIB encompasses a wide spectrum of symptoms, ranging from trivial hematochezia to massive hemorrhage with shock. Acute LGIB is defined as bleeding that is of recent duration, originates beyond the ligament of Treitz, results in instability of vital signs, and is associated with signs of anemia with or without need for blood transfusion.
LGIB is classified under 3 groups according to the amount of bleeding, as shown in the image below. Massive hemorrhage is a life-threatening condition and requires transfusion of at least 5 U of blood.
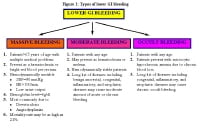 Types of lower gastrointestinal (GI) bleeding. HR = heart rate; SBP = systolic blood pressure.
Types of lower gastrointestinal (GI) bleeding. HR = heart rate; SBP = systolic blood pressure.
Massive lower GI bleeding is defined as follows:
Passage of a large volume of red or maroon blood through the rectum
Hemodynamic instability and shock
Initial decrease in hematocrit (Hct) level of 6 g/dL or less
Transfusion of at least 2 U of packed red blood cells (RBCs)
Bleeding that continues for 3 days
Significant rebleeding in 1 week
LGIB has a mortality rate ranging from about 10-20%, with patients of advanced age (>60 y) and patients with comorbid conditions (eg, multiorgan system disease, transfusion requirements in excess of 5 units [U], need for operation, and recent stress, such as surgery, trauma, and sepsis) at greatest risk. LGIB is more likely in the elderly because of a higher incidence of diverticulosis and vascular disease in these groups. The incidence of LGIB is higher in men than in women.
Advances in diagnostic and therapeutic
colonoscopy and in interventional angiography have resulted in a shift away from the need for surgical treatment (see the image below). Effective management with less invasive modalities has also reduced healthcare costs and, more importantly, patient morbidity and mortality.
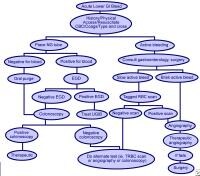 Methods used to treat lower gastrointestinal bleeding (LGIB).
Methods used to treat lower gastrointestinal bleeding (LGIB).
Historical details
Understanding of the pathogenesis, diagnosis, and treatment of LGIB has drastically changed during the last 50 years. In the first half of the 20th century, large intestinal neoplasms were believed to be the most common cause of LGIB. In the 1950s, this condition was commonly attributed to diverticulosis; surgical treatment consisted of blind segmental bowel resections, with disappointing results. Patients who underwent these procedures suffered from a prohibitively high rebleeding rate (up to 75%), morbidity (up to 83%), and mortality (up to 60%).
In the last 4 decades, diagnostic methods for locating the precise bleeding point greatly improved. The flexible endoscope was developed in 1954. The full-length colonoscope was developed in 1965 in Japan. Also in 1965, Baum et al described selective mesenteric angiography, which permitted the identification of vascular abnormalities and the precise bleeding point.
[2] The first anal colonoscopy was performed in 1969.
Experience with mesenteric angiography in the late 1960s and 1970s suggested that angiodysplasias and diverticulosis were the most common reasons for LGIB. Since its discovery, mesenteric angiography remains the criterion standard in precise localization of the bleeding.
Rosch et al described superselective visceral arteriography for infusion of vasoconstrictors in 1971 and superselective embolization of the mesenteric vessels as an alternative technique to treat massive LGIB in 1972.
[3, 4] The most feared complication of embolization of the mesenteric vessels is ischemic colitis, which has limited its use for GI bleeding.
The initial experience with vasopressin infusion was reported in 1973-1974. Vasopressin causes vasoconstriction and arrests the bleeding in 36-100% of patients. The recurrence rate following completion of vasopressin infusion can be as high as 71%; therefore, vasopressin is used to temporize the acute event and to stabilize patients before surgery.
Endoscopic control of bleeding with thermal modalities or sclerosing agents has been in use since the 1980s. One of the advantages of upper (or lower) endoscopic evaluation is that it provides a means to administer therapy in patients with GI bleeding. Nuclear scintigraphy has been used since the early 1980s as a very sensitive diagnostic tool to evaluate bleeding from GI tract; this modality can detect hemorrhage at rates as low as 0.1 mL/min
 Distal common bile duct tumor excised by radical pancreaticoduodenectomy. The tumor measured 1.2 cm in diameter.
Distal common bile duct tumor excised by radical pancreaticoduodenectomy. The tumor measured 1.2 cm in diameter.  Distal common bile duct tumor excised by radical pancreaticoduodenectomy. The tumor measured 1.2 cm in diameter.
Distal common bile duct tumor excised by radical pancreaticoduodenectomy. The tumor measured 1.2 cm in diameter.